Loading...
Loading...

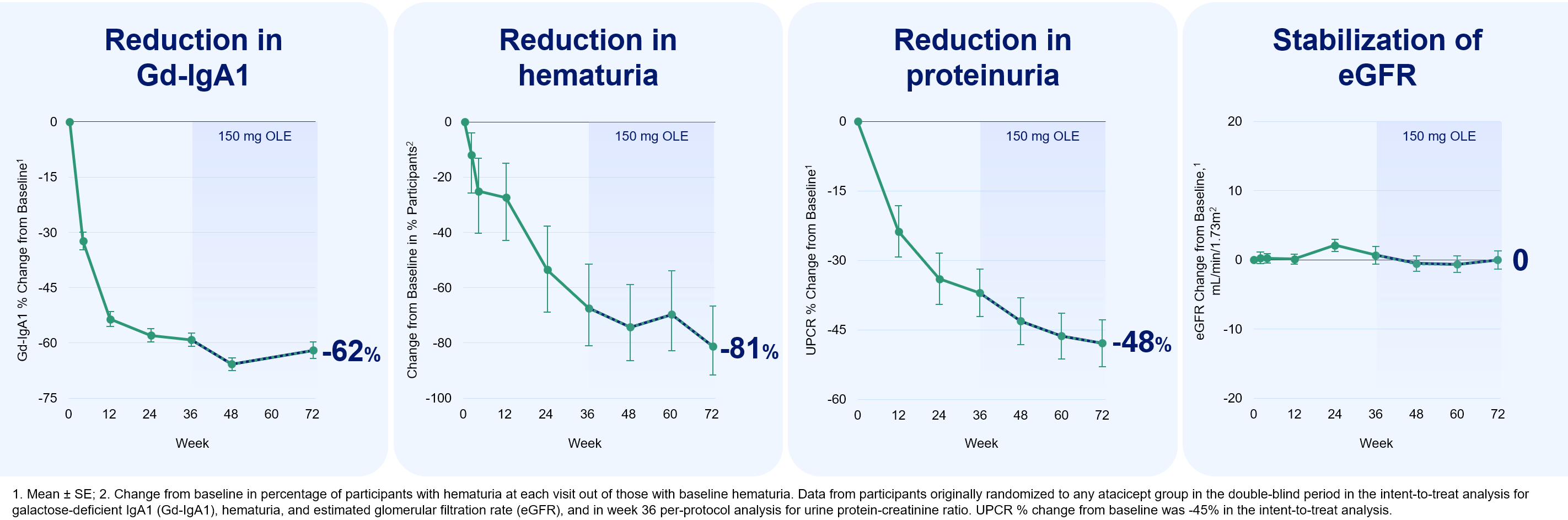
72-week data consistent with disease modification in IgAN, selected as a best-ranked abstract; Rapid and sustained improvements in hematuria over 36 weeks, with resolution in significantly greater percentage of participants than placebo; BRISBANE, Calif., May 25, 2024 (GLOBE NEWSWIRE) -- Vera Therapeutics, Inc. VERA , a late clinical-stage biotechnology company focused on developing and commercializing transformative treatments for patients with serious immunological diseases, today announced data presentations from its Phase 2b ORIGIN trial of atacicept in immunoglobulin A nephropathy (IgAN), showing that atacicept stabilized kidney function through 72 weeks and led to rapid improvements in hematuria.
These data were presented at the 61st European Renal Association Congress (ERA24) being held in Stockholm. "For the first time in this field, we presented 72-week data from our Phase 2b ORIGIN trial showing stable kidney function over the duration of treatment. In addition, we presented data showing that atacicept leads to hematuria resolution in significantly more patients compared with placebo.
The impact on hematuria was seen as early as 4 weeks after treatment initiation, which could have important implications for patients with acute kidney inflammation. The evolving atacicept data package supports our belief that atacicept may offer comprehensive disease modification to patients with IgAN," said Marshall Fordyce, M.D.
, Founder and CEO of Vera Therape.