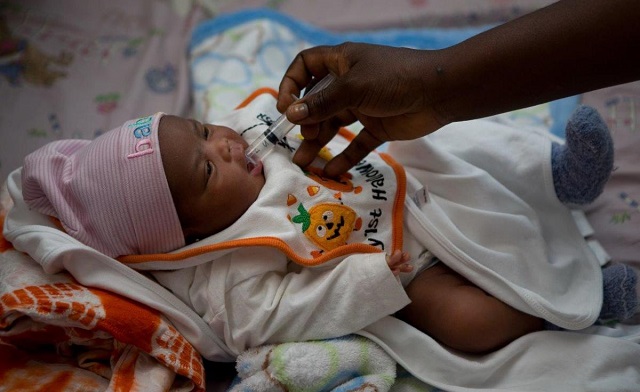
Researchers in Uganda are making significant strides in the fight against pediatric HIV. Leveraging data from the International Maternal, Pediatric, Adolescent AIDS Clinical Trials (IMPAACT P1115) study, they are exploring the complexities of Analytical Treatment Interruptions (ATIs) as a potential path towards a cure. IMPAACT P1115 is an ongoing, phase 1/2, proof-of-concept study in which infants were enrolled at 30 research clinics in 11 countries (Brazil, Haiti, Kenya, Malawi, South Africa, Tanzania, Thailand, Uganda, the USA, Zambia, and Zimbabwe) into two cohorts.
Despite substantial advances in preventing perinatal HIV transmission, infants remain at high risk for HIV due to undiagnosed or untreated maternal HIV. Babies born with HIV have limited treatment options, as HIV medications approved for new-borns can be challenging to give consistently, usually once or twice per day. Findings published by IMPAACT P11115 research show that for newborns with HIV, starting treatment within 48 hours of birth is feasible and safe.

Current findings indicate that very early treatment limits the build-up of HIV reservoirs. For some babies, this may help make remission achievable. Remission means having no HIV detectable in blood tests after treatment is stopped.
One promising strategy involves Analytical Treatment Interruptions (ATIs). ATIs are usually used in HIV cure-related trials to see whether an investigational HIV drug or strategy can delay or prevent viral rebound after study .