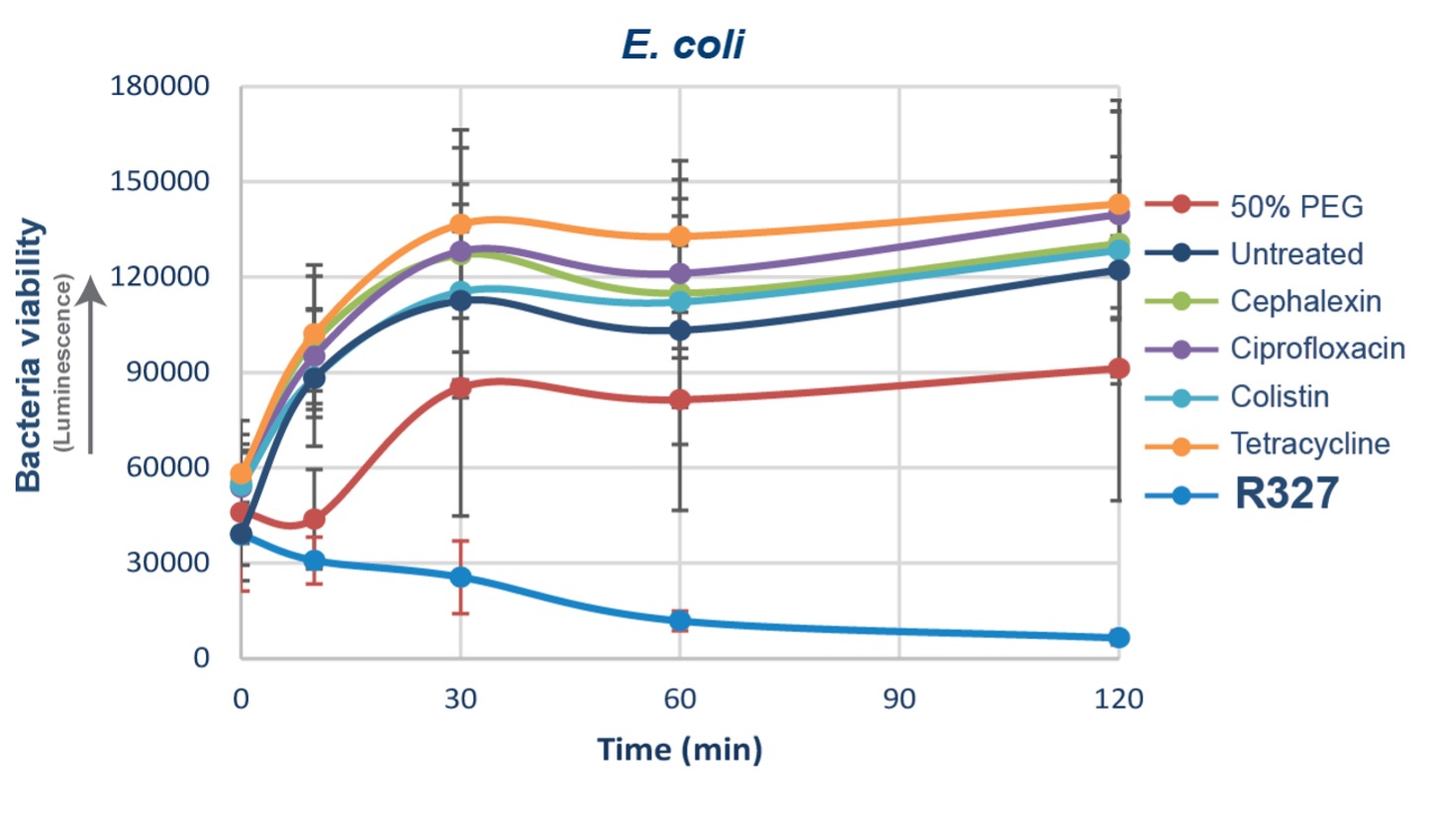
SYDNEY, July 01, 2024 (GLOBE NEWSWIRE) -- Recce Pharmaceuticals Ltd (ASX: RCE, FSE: R9Q) (the Company), the Company developing a new class of synthetic anti-infectives, today announced positive data from a Phase I/II trial for urinary tract infections (UTIs) and urosepsis, demonstrating that its lead candidate, RECCE 327 (R327), administered intravenously is safe and efficacious against ( ). “The positive outcomes from this clinical trial provide more evidence of R327 as rapid-acting for the treatment of serious and life-threatening bacterial infections,” said Alan Dunton, MD, Chief Medical Advisor at Recce Pharmaceuticals. “The ability of R327 to disrupt bacterial energy production so effectively and sustain its activity over several hours highlights its potential as a transformative treatment for serious and/or resistant bacterial infections, including complicated UTIs/.
The mechanism is novel as an antibacterial, which has been proven safe in humans. We are excited to further explore these findings and advance R327 through subsequent trial phases.” Marc Sharp, Ph.

D., Chief Scientific Officer of Linnaeus Bioscience, added, “The ability of R327 to achieve biologically relevant concentrations and exhibit anti-bacterial activity in urine samples is highly encouraging.” The Phase I/II study included 25 healthy participants who received R327 at doses up to 4,000mg as intravenous infusions over various infusion times (15, 20, 30, and 45 minutes).
The highest dose coho.