REHOVOT, Israel, June 27, 2024 (GLOBE NEWSWIRE) -- (“Purple Biotech” or “the Company”) (NASDAQ/TASE: PPBT), a clinical-stage company developing first-in-class oncology therapies that overcome tumor immune evasion and drug resistance, today reported additional positive interim data from its randomized, controlled, open label, multicenter Phase 2 study ( ) of CM24, in combination with Bristol Myers Squibb’s immune checkpoint inhibitor nivolumab and standard of care (SoC) chemotherapy, in second-line metastatic pancreatic ductal adenocarcinoma (PDAC). These results suggest that serum MPO may be a predictive biomarker for survival in the CM24+Nivolumab + Nal-IRI/5FU/LV arm. The company also announced that it will host a virtual KOL event on Thursday, July 11, 2024 at 10:30 AM ET to discuss the results in detail.
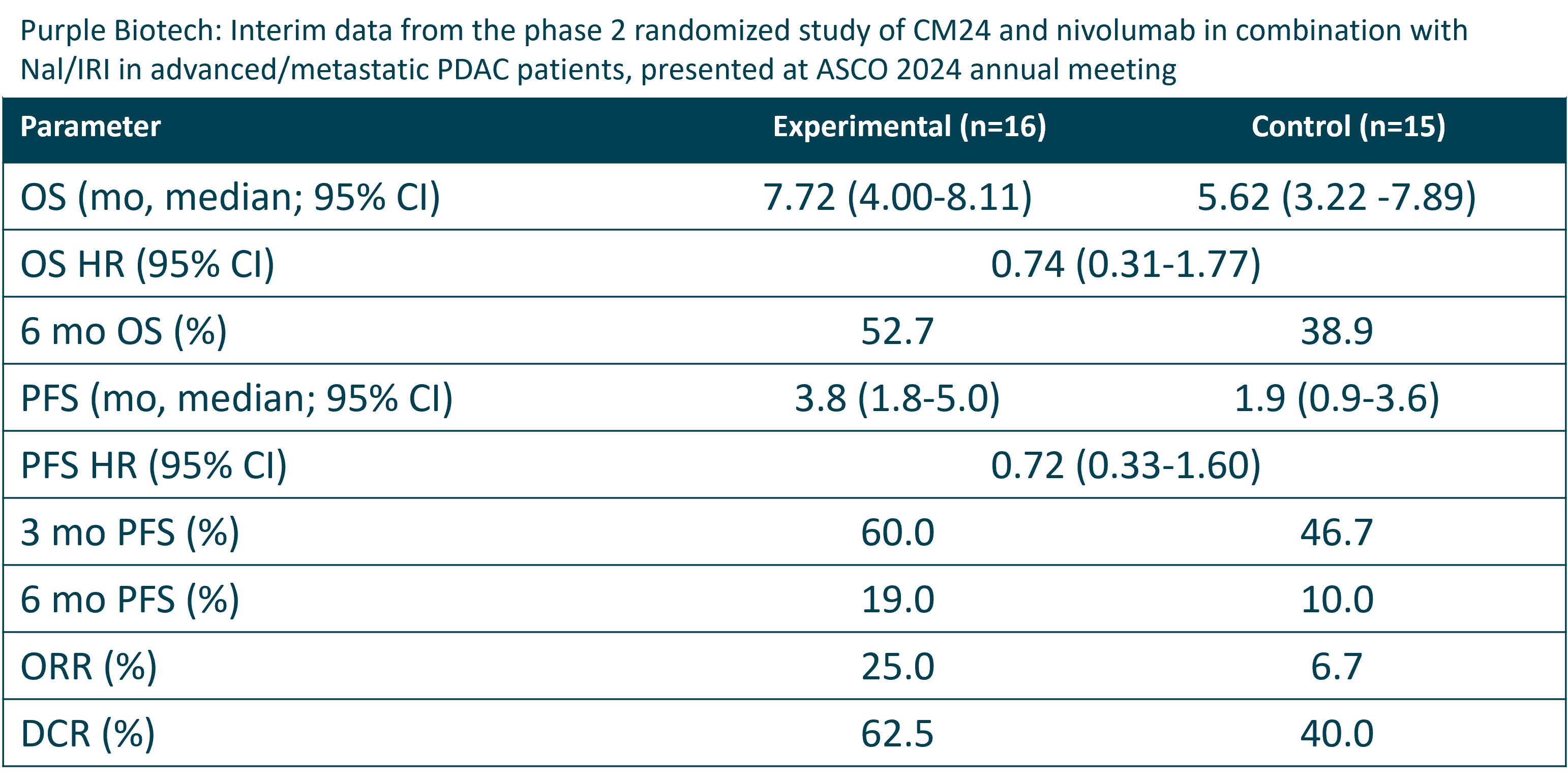
To register for the event, Interim results that were presented on June 1, 2024 during a late-breaking abstract poster presentation at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting demonstrated a 26% reduction in risk of death (HR=0.74) and a 28% risk reduction in progression or death (HR=0.72) in previously-treated patients treated with CM24+nivolumab+Nal-IRI/5FU/LV vs.

Nal-IRI/5FU/LV chemotherapy alone (i.e., SoC).
Median OS was prolonged by 2.1 months and median PFS was extended by 1.9 months in the CM24+nivolumab+Nal-IRI/5FU/LV regimen vs.
SoC. The prolongation of both OS and PFS by the CM-24-nivolumab therapy is further supported by a .