PRINCETON, N.J., June 12, 2024 (GLOBE NEWSWIRE) -- PDS Biotechnology Corporation (Nasdaq: PDSB) (“PDS Biotech” or the “Company”), a late-stage immunotherapy company focused on transforming how the immune system targets and kills cancers and the development of infectious disease vaccines, today provided a data update from its ongoing VERSATILE-002 Phase 2 clinical trial.
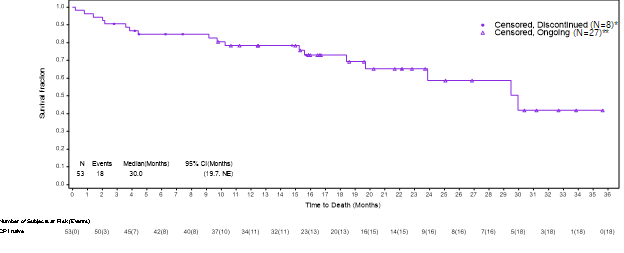
VERSATILE-002 is evaluating Versamune HPV + KEYTRUDA (pembrolizumab) in patients with HPV16-positive head and neck squamous cell cancer (“HNSCC”). The Kaplan-Meier analysis described below captures the survival data for immune checkpoint inhibitor (“ICI”) naïve patients from the ongoing VERSATILE-002 Phase 2 clinical trial. Based on a data cut as of May 17, 2024, the updated survival data for the cohort of ICI naïve patients after an additional follow-up of approximately 6 months in the VERSATILE-02 Phase 2 clinical trial with a total of 53 enrolled patients was as follows: Full data from the May 17, 2024 data cut are expected to be announced in Q3 2024.

Dr. Kirk Shepard, M.D.
, Chief Medical Officer of PDS Biotech stated, “In recurrent and/or metastatic HNSCC, objective response rate and progression-free survival have generally not translated into increased survival, and under current standards of care survival rates are well established to be less than 18 months. We believe that our VERSATILE-002 clinical trial and triple combination trial provide us with the critical survival informatio.