Loading...
Loading...

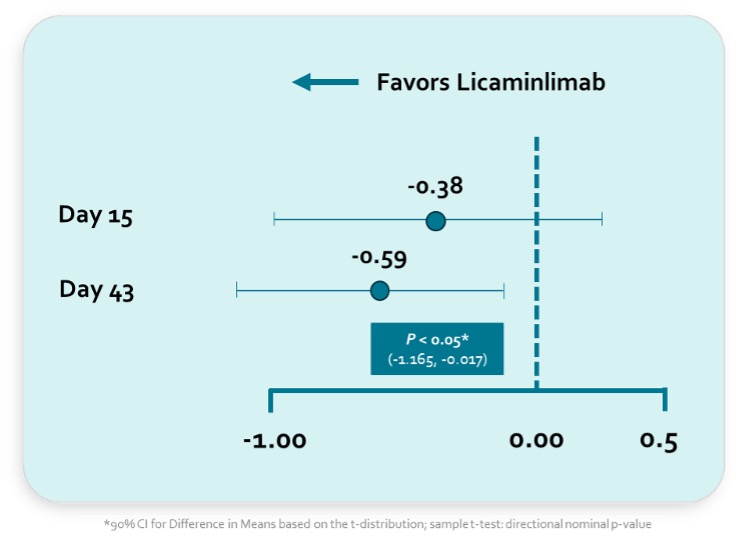
Improvements in multiple sign efficacy endpoints were observed in full population and with predictive and more pronounced effects in the TNFR1 genetic biomarker population as identified in prior successful Phase 2 symptoms trial Rapid treatment effect on corneal inflammation was observed in TNFR1 genetic biomarker patients as early as Day 15 and was statistically significant at final efficacy visit on Day 43 Licaminlimab was well tolerated similar to vehicle Company plans to finalize Phase 3 development plans following an End-of-Phase 2 (EoP2) meeting with the U.S. Food and Drug Administration (FDA) An investor and analyst webcast will be held today at 8:30am US Eastern Time ZUG, Switzerland, June 10, 2024 (GLOBE NEWSWIRE) -- Oculis Holding AG OCS ("Oculis"), a global biopharmaceutical company purposefully driven to save sight and improve eye care, today announced positive topline results from its Phase 2b RELIEF trial with licaminlimab, a novel anti-TNFα biologic eye drop with an established dual anti-inflammatory and anti-apoptotic mechanism of action in patients with dry eye disease (DED).
The Phase 2b RELIEF trial is a multi-center, randomized, double-masked, vehicle-controlled trial evaluating the efficacy and safety of licaminlimab in subjects with signs of DED ( NCT05896670 ). The trial also evaluated the efficacy and safety of licaminlimab in a subpopulation of subjects with a TNFR1-related genotype as prespecified in the protocol. One hundred a.