DANBURY, Conn. and WESTLAKE VILLAGE, Calif., June 22, 2024 (GLOBE NEWSWIRE) -- , a company focused on the development and commercialization of inhaled therapeutic products and devices for patients with endocrine and orphan lung diseases, today announced positive 17-week results from the INHALE-3 study, a Phase 4 U.
S. clinical trial evaluating Afrezza (plus basal insulin) vs. usual care (defined as multiple daily injections (MDI), an automated insulin delivery system, (AID) or a pump without automation) utilizing a higher initial conversion dose from mealtime injectable insulin to inhaled insulin.

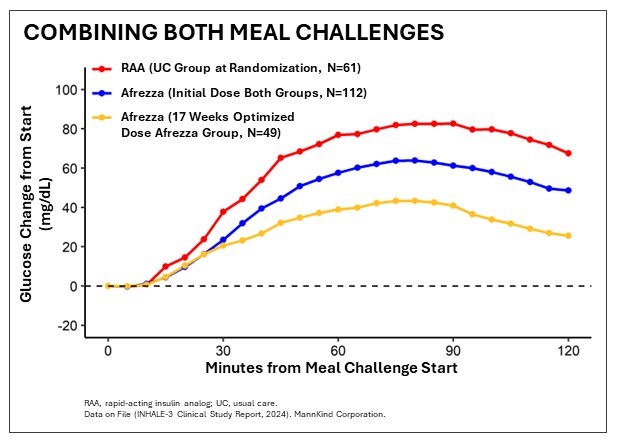
The study, which was presented by the INHALE-3 investigational team at the American Diabetes Association’s (ADA) 84 Scientific Sessions in Orlando, met its primary efficacy endpoint of a non-inferior change in HbA1c between baseline and week 17 compared to the usual care group. Key sub-analysis findings included: “Inhaled insulin demonstrated improved mealtime control, which is significant given how this continues to be a significant unmet need,” said Dr. Irl Hirsch, Professor of Medicine and Diabetes Treatment and Teaching Chair at the University of Washington and the INHALE-3 Study Protocol Chair.
“The INHALE-3 study delivered data that supports inhaled insulin being an important treatment option for adults living with diabetes.” “INHALE-3 adds to the body of evidence that when combined with basal insulin, inhaled insulin’s effect on HbA1c/TIR is similar to that of th.