, /PRNewswire/ -- Biohaven Ltd. (NYSE: ) (Biohaven), a global clinical-stage biopharmaceutical company focused on the discovery, development and commercialization of life-changing therapies to treat a broad range of rare and common diseases, announced the first patient has been dosed in a first-in-human Phase 1/2 study of BHV-1510, a highly differentiated Trophoblast Cell Surface Antigen-2 (Trop-2) directed Antibody Drug Conjugate (ADC), and the lead ADC program to advance into clinical trials in Biohaven's growing oncology pipeline. The Phase 1/2 study of BHV-1510 is a multicenter, open-label study in subjects with select advanced or metastatic epithelial cell tumors.
The trial consists of a dose-escalation phase, followed by a multicohort expansion phase. Additional information can be found at (NCT06384807). "We are extremely proud to advance our first oncology clinical program with a potentially best in class ADC," said , M.

D., Chief Medical Officer of Oncology at Biohaven. "With the initiation of this monotherapy study, we are one step closer to providing differentiated and superior treatment options to people living with cancer.
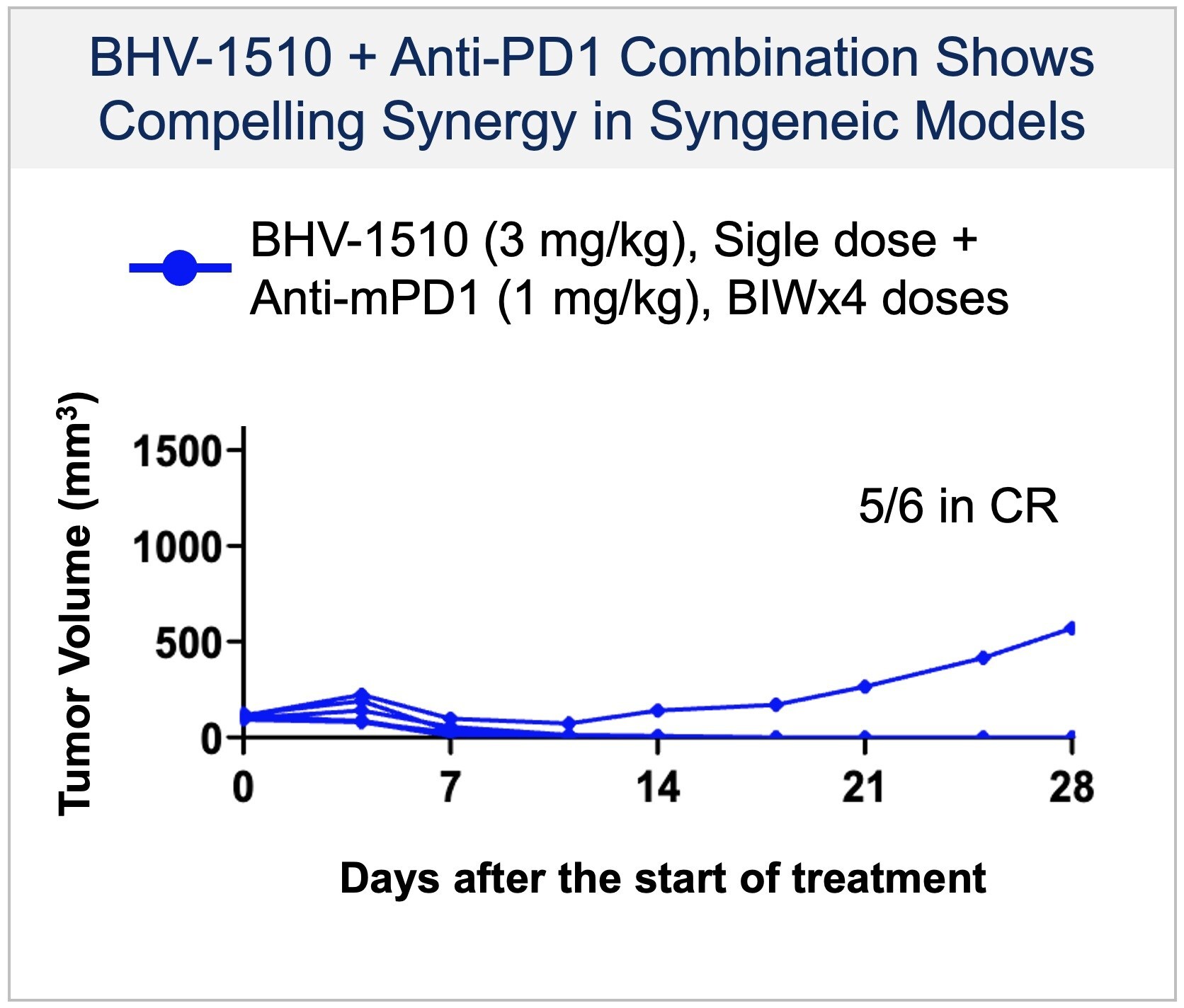
We are also excited to work with Regeneron and efficiently explore BHV-1510 in combination with its PD-1 inhibitor across a range of tumors." BHV-1510 is a next-generation, fully optimized ADC that consists of a Trop-2 directed antibody conjugated to a proprietary best-in-class Topoisomerase 1 (TopoIx) payload at a homogeneous drug-antibody ratio.