Loading...
Loading...

VALENCIA, Calif., May 30, 2024 (GLOBE NEWSWIRE) -- AVITA Medical, Inc. RCEL AVH )), a commercial-stage regenerative medicine company focused on first-in-class devices for wound care management and skin restoration, today announced that the U.
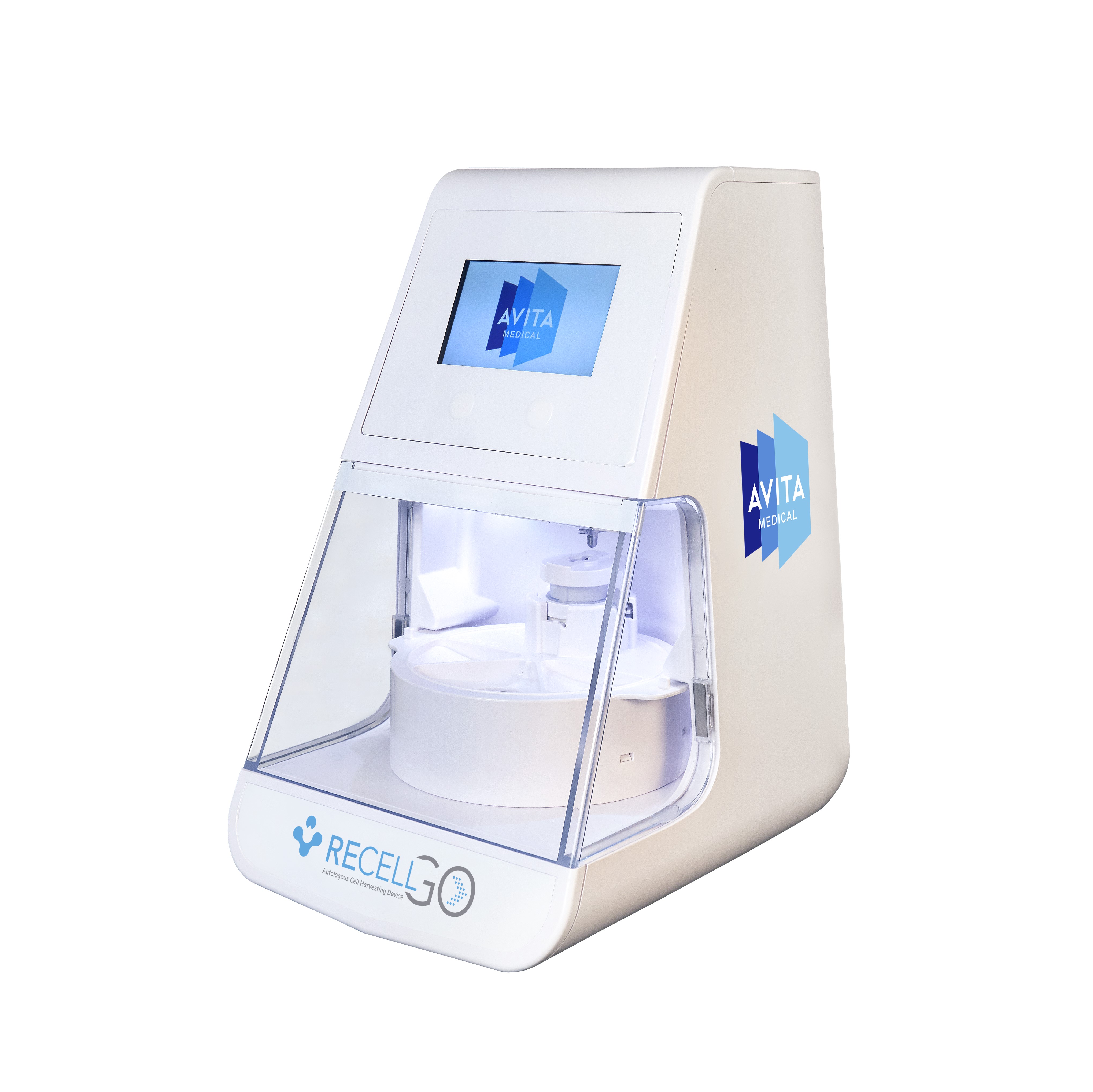
S. Food and Drug Administration (FDA) has approved its premarket approval (PMA) supplement for the RECELL GO TM System, its next-generation autologous cell harvesting device that harnesses the regenerative properties of a patient's own skin to treat thermal burn wounds and full-thickness skin defects. When choosing RECELL, clinicians and patients can realize several significant advantages over traditional skin grafting: Improved healing is achieved using significantly less donor skin 1 Pain is reduced, closure is faster, and the aesthetic appearance at the RECELL-harvested donor site is improved 2 Fewer procedures are required for definitive closure 3 There's a reduction in the length of stay for burns covering less than 50% Total Body Surface Area (TBSA) 2 , 3 , 4 RECELL GO introduces enhanced features that streamline the preparation of Spray-On Skin TM Cells.
This next-generation device significantly reduces the training burden on medical staff, improves workflow efficiency in the operating room, and controls the RECELL Enzyme TM incubation time to ensure optimal cell yield and viability. These advancements simplify the user interface, enabling medical teams to provide quality care readily and consistently to their.