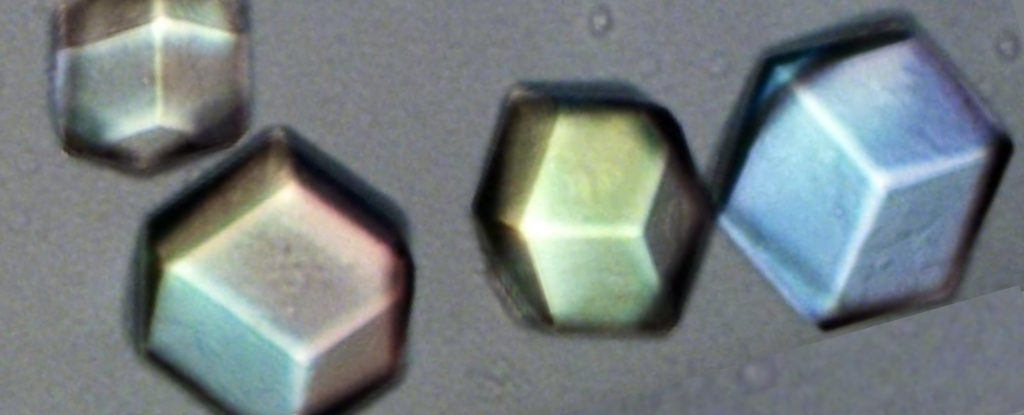
A new technique that produces 3D models of individual crystals has opened a window for scientists to see the subtle deviations that emerge in their otherwise perfect patterns. Researchers from New York University (NYU) went back to the drawing board on how to look deep inside solids made of repeating units, and determine how they grow. With a short wavelength roughly the same size as many of the repeating units that make up crystals, X-rays have long allowed scientists to infer how a crystal's components fit together by measuring the angle at which the rays are diffracted.
For all its ingenuity, though, X-ray crystallography has its limits, which are summed up rather neatly by the opening sentence of a new paper published in this month: "Structures of molecular crystals are identified using scattering techniques because we cannot see inside them." The paper describes a new technique that promises to finally change that fact – albeit not for crystals composed of repeating units of individual atoms. Instead, it concerns crystals composed of patterns based on , which are large enough to be seen under a conventional microscope and to manipulate in a manner that would be impossible for atoms.

Studying such crystals has allowed for advances in the understanding of crystal dynamics. The researchers cite experiments with colloidal structures that shed light on the and of dislocations within crystal structures. Like X-ray crystallography, this technique has limits.
Difficulties in f.